8 Nonferrous Metals Hiding in Plain Sight Around Your Home
Did you know your house is full of valuable nonferrous metals? You probably handle them every single day without a second thought. From the coins in your pocket to the lightweight frame of your eyeglasses, these essential materials are the building blocks of modern life. They are hiding in plain sight, making your world safer, more efficient, and more connected. This guide reveals 8 examples of these hidden treasures, showing you exactly where they are and why they are so important.
Table of Contents
Before we start our treasure hunt, let’s quickly define our terms. When we talk about metals, they includes two groups: ferrous and nonferrous.
The difference is simple: iron.
- Ferrous metals contain iron. The most common examples are steel and cast iron. You know them well—they are heavy, strong, and magnetic.
- Nonferrous metals, on the other hand, do not contain any significant amount of iron. This simple difference gives them a whole range of unique and highly desirable properties. They are generally:
- Not magnetic.
- More resistant to rust and corrosion.
- Lighter in weight.
- Excellent conductors of heat and electricity.
These characteristics make them essential for everything from electrical wiring to aerospace engineering. At ZONEDING, our expertise begins at the source. We provide the heavy-duty equipment like Jaw Crushers and Ball Mills that crush and grind the raw ore, which is the very first step in liberating these valuable metals from the rock.
Now, let’s explore where these amazing materials are hiding around your home. You are surprising by how common they are.
1. Copper (Cu)
- Where you’ll find it: Electrical wiring inside your walls, plumbing pipes, the bottoms of high-end cookware, and the windings inside motors in your appliances.
- Why use it: Copper’s superpower is its phenomenal electrical conductivity, second only to silver. It allows electricity to flow with very little resistance, which is why it’s the global standard for wiring. It’s also highly resistant to corrosion, making it perfect for water pipes that need to last for decades.
- Applications: Beyond homes, copper is the backbone of the energy and transportation sectors. It’s in industrial motors, power transformers, and generators. The transition to green energy heavily relies on copper for wind turbines and electric vehicle (EV) motors and batteries, which use several times more copper than conventional cars.
- From the mine: Copper comes from ores like chalcopyrite. After crushing, the ore is ground into a fine powder and then goes through a Flotation Machine, which uses bubbles to separate the tiny copper-bearing particles from the waste rock.
2. Aluminum (Al)
- Where you’ll find it: Soda cans, kitchen foil, window frames, pots and pans, and the bodies of modern laptops and smartphones.
- Why use it : Aluminum is the champion of being lightweight yet strong. It has an excellent strength-to-weight ratio, which is why it’s in everything from beverage cans to aircraft. It also naturally forms a protective oxide layer, making it very corrosion-resistant.
- Applications: The aerospace and automotive industries are massive consumers of aluminum. It applies for aircraft fuselages and engine components to reduce weight and save fuel. In cars, it’s for engine blocks, wheels, and body panels. It’s also for high-voltage overhead power lines because it’s lighter than copper.
- From the mine: Aluminum originates from bauxite ore. The process to refine it is complex and energy-intensive, but it all starts with crushing the raw bauxite to a manageable size for processing.
3. Zinc (Zn)
- Where you’ll find it: This one is a bit more difficult to find. It’s the primary component in many alkaline batteries (like AA and AAA), and it’s as a protective coating on steel in a process called galvanization. Zinc can be in your steel nails, screws, and outdoor metal fences to prevent rust.
- Why use it : Zinc has a unique property: it corrodes in preference to steel. When used as a coating, it acts as a sacrificial layer. Any rust will attack the zinc first, protecting the steel underneath for many years.
- Applications: Its main industrial use is galvanizing steel for construction and infrastructure projects. Zinc is also a crucial ingredient in making brass (an alloy of copper and zinc) and is in die-casting to create intricate parts for car engines and door handles. Zinc oxide is a key ingredient in rubber manufacturing and is also in sunscreens and lotions.
- From the mine: Zinc is usually extracted from the mineral sphalerite, often found alongside lead and copper ores.
4. Nickel (Ni)
- Where you’ll find it: Coins (like the U.S. nickel), stainless steel sinks and cutlery, and rechargeable batteries in your power tools and older electronics.
- Why use it : Nickel’s main job is to make other metals better. When added to steel, it creates stainless steel, which is incredibly resistant to rust, stains, and corrosion. This toughness and shiny finish make it ideal for kitchen applications.
- Applications: Nickel is a critical material for high-performance applications. It’s a key component of superalloys to make jet engine turbine blades that can withstand extreme temperatures. It is also an essential cathode material in the lithium-ion batteries that power the vast majority of electric vehicles today (e.g., NMC and NCA chemistries).
- From the mine: Nickel ores like pentlandite and laterites require a variety of processing techniques, often involving crushing, grinding, and complex pyrometallurgical or hydrometallurgical processes to isolate the metal.
5. Titanium (Ti)
- Where you’ll find it: High-end eyeglass frames, lightweight camping gear, premium golf clubs, and some laptop cases. It also widely applies as titanium dioxide, a brilliant white pigment in your house paint, sunscreen, and even toothpaste.
- Why use it : Titanium has the highest strength-to-density ratio of any metallic element. This means it is as strong as some steels but 45% lighter. It is also exceptionally corrosion-resistant and hypoallergenic.
- Applications: Its unique properties make it invaluable in aerospace for structural aiframe components, landing gear, and jet engines. In the medical field, it’s the gold standard for surgical implants like hip and knee replacements and dental implants because the human body does not reject it. It is also in chemical processing plants where equipment faces highly corrosive substances.
- From the mine: Titanium is extracted from minerals like ilmenite and rutile, which are often found in mineral sands. Processing these sands involves advanced separation techniques using gravity and Magnetic Separators to concentrate the heavy titanium-bearing minerals.
6. Tin (Sn)
- Where you’ll find it: The solder used to join electronic components on circuit boards inside your TV, computer, and phone. It’s also as a non-corrosive coating on steel food cans.
- Why Use it: Tin has a low melting point, which makes it perfect for creating solder. It can be easily melted to form strong electrical connections and then quickly solidify. Its non-toxic and corrosion-resistant nature made it the historical choice for protecting food cans.
- Applications: Beyond electronics, tin is a key component of many useful alloys, including bronze (tin and copper) and pewter. A huge amount of tin is in the Pilkington process to create perfectly flat window glass by floating molten glass on a bed of molten tin. It is also in a range of chemical compounds for PVC plastics and fungicides.
- From the mine: The primary source of tin is the mineral cassiterite. Processing often involves gravity concentration methods, like using shaking tables, to separate the heavy cassiterite from lighter minerals after initial crushing and grinding.
7. Lead (Pb)
- Where you’ll find it: The primary place you’ll find lead today is inside a car battery (lead-acid battery). Due to its toxicity, its use in homes has been drastically reduced, but older homes may still have lead pipes or lead-based paint.
- Why use it: Lead is extremely dense, soft, and malleable, and its ability to store and deliver large electrical currents makes it ideal for car batteries, which remain the most cost-effective and reliable option for starting internal combustion engines.
- Applications: The vast majority of lead (over 80%) is for lead-acid batteries for vehicles and backup power supplies. Its extreme density makes it an unparalleled material for radiation shielding in hospitals (X-ray rooms, PET scanners), nuclear power plants, and industrial radiography. It is in ammunition and as a weight for balancing tires.
- From the mine: Lead is mainly extracted from galena ore. It is one of the easiest metals to smelt.
8. Magnesium (Mg)
- Where you’ll find it: The bodies of some high-end cameras, power tools, and laptops. It is often as an alloy to make them strong but feather-light.
- Why use it: Magnesium is the lightest of all structural metals, about 30% lighter than aluminum. When alloyed with other elements, it can achieve a strength comparable to some aluminum alloys, making it the perfect choice when every gram counts.
- Applications: The automotive industry uses magnesium alloys to reduce vehicle weight in parts like steering wheel frames, seat frames, and gearbox casings, which helps improve fuel efficiency. Its bright, white flame when burned makes it a key ingredient in fireworks and emergency flares. It is also in metallurgy to remove sulfur from iron and steel.
- From the mine: Magnesium is from seawater, brines, and magnesium-bearing minerals like magnesite and dolomite.
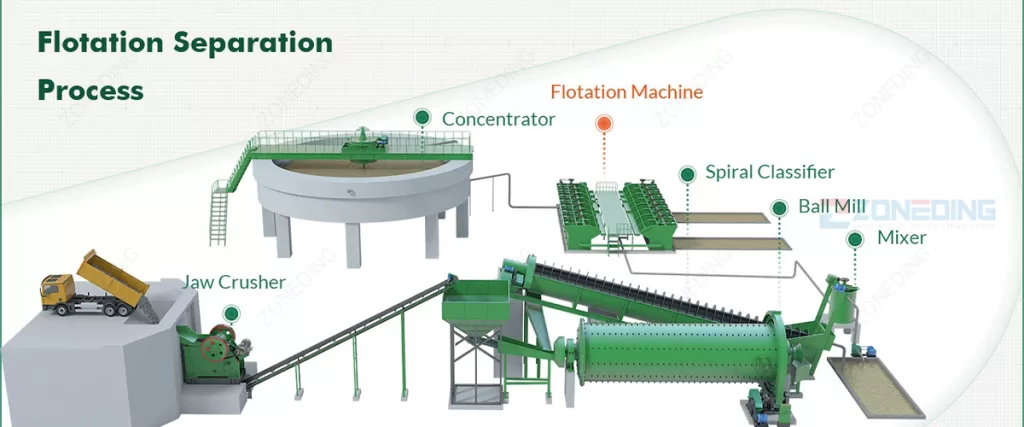
From Mine to Your Home: A Quick Look
As you can see from the equipment above, every nonferrous metal in your home started its journey deep within the earth as a mineral locked inside ore. Getting it from rock to a usable product is the science of mineral processing. The general path looks like this:
- Mining: Large equipment extracts the ore-bearing rock from the ground.
- Comminution (Crushing & Grinding): The rock went to a processing plant where our crushers and ball mills perform the heavy lifting of size reduction to liberate the minerals.
- Separation: The valuable minerals are separated from the waste rock (tailings) using specialized techniques and equipment like flotation cells and magnetic separators.
- Refining: The concentrated minerals are then smelted or refined through chemical processes to produce the pure metal you see in your everyday products.
What is the Equipment in This Process?
Getting a pure metal like copper from a giant, rugged piece of rock is an industrial marvel. It requires a sequence of powerful and precise machines working together in a processing plant. At ZONEDING, we manufacture the key equipment that makes this transformation possible.
Stage 1: Comminution (Crushing and Grinding)
The first step is to break down the huge boulders from the mine into a fine powder. This is “comminution,” and this stages include:
- Primary Crushing: The process starts with a Jaw Crusher. This machine acts like a giant mechanical jaw, using immense force to break rocks that can be over a meter wide down to the size of your fist.
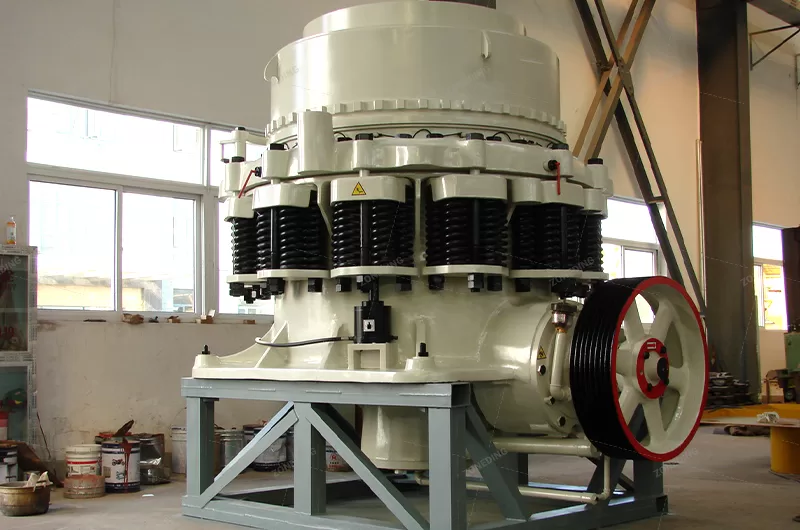
- Secondary and Tertiary Crushing: The output from the jaw crusher then goes to a Cone Crusher. This machine grinds the rock further, reducing it to the size of gravel. This might be done in one or two stages to get the size just right for the next step.
- Grinding: The final and most critical stage of comminution happens in a Ball Mill. This is a huge rotating drum filled with steel balls. As the drum turns, the steel balls tumble and crush the gravel into a fine, flour-like powder. This step is essential to “liberate” the microscopic particles of the valuable metal from the waste rock.
Stage 2: Separation and Concentration
Once the metal is liberated, you have to separate it from all the worthless powder. This is done using equipment that exploits the unique physical and chemical properties of the metal:
- Flotation: For metals like copper, zinc, and lead, we use Flotation Machines. The powder is mixed with water and special reagents in large tanks. Air is bubbled through the mixture, and the chemical reagents make only the valuable metal particles stick to the bubbles. The metallic froth floats to the top and is skimmed off, while the waste rock sinks.
- Magnetic Separation: For minerals that are magnetic (or have magnetic components), we use a Magnetic Separator. A powerful magnet pulls the target minerals away from the non-magnetic waste. This is common in processing titanium ores.
- Gravity Separation: For very dense minerals like tin, gravity can be used. A Shaking Table is a tilted, vibrating platform. When the ore slurry flows over it, the heavy tin particles get caught in ridges on the table while the lighter waste rock washes away.
Why This Matters for the Future
Understanding the nonferrous metals around you is more than just a fun fact. As we move toward a greener, more technologically advanced future, the demand for these metals is soaring. Copper is essential for electric vehicles and renewable energy grids. Aluminum and titanium are key to making transportation more fuel-efficient. And a whole host of others are critical for our electronics.
This highlights the B2growing importance of two things: efficient and responsible primary extraction, and the recycling of these materials. Because nonferrous metals don’t degrade during recycling, they can be used over and over again, saving enormous amounts of energy and reducing the need for new mining.
FAQ
- 1. Are nonferrous metals valuable for recycling?
- Yes, extremely. Metals like copper and aluminum have a high scrap value because recycling them uses up to 95% less energy than producing them from raw ore. Collecting and recycling these metals is both economically and environmentally beneficial.
- 2. Is stainless steel a nonferrous metal?
- This is a common point of confusion. Stainless steel is a ferrous metal because its primary component is iron. However, it gets its “stainless” properties from nonferrous metals like nickel and chromium being added to it.
- 3. How can I tell if a metal is nonferrous?
- The easiest home test is with a magnet. If a magnet sticks to it strongly, it’s a ferrous metal. If it doesn’t stick at all, it’s nonferrous. (Note: Some stainless steels are weakly magnetic, but most nonferrous metals show no attraction).
- 4. Why is gold considered a nonferrous metal?
- Gold (Au) is a nonferrous metal because, by definition, it does not contain iron. It belongs to the “precious metals” sub-category of nonferrous metals, along with silver, platinum, and palladium.
Conclusion
The next time you flip a light switch, drink from a can, or put on your glasses, take a moment to appreciate the incredible journey of the nonferrous metals that make it all possible. They began as humble minerals, were liberated from rock by powerful machinery, and were refined into the high-performance materials that quietly power our modern world. These metals aren’t just hiding in your home; they are actively working to make your life better.
At ZONEDING, we are proud to be at the very start of this journey, providing the foundational equipment that helps unlock these essential resources for the world.